To sporulate or not to sporulate
 When nutrients are scarce, Bacillus subtilis cells are able to form highly resistant endospores. However, even in a clonal population, only some cells engage in the sporulation process. This is explained in terms of a bet-hedging strategy: a mixed population composed of both vegetative cells and spores is prepared for a variety of unknown future environments. But how does an individual cell determine its own fate? In a recent report, J. W. Veening et al. showed that the decision (to sporulate or not to sporulate) is not taken by the cell itself ― it is determined a few generations earlier, and the command is inherited as a “memory”.
When nutrients are scarce, Bacillus subtilis cells are able to form highly resistant endospores. However, even in a clonal population, only some cells engage in the sporulation process. This is explained in terms of a bet-hedging strategy: a mixed population composed of both vegetative cells and spores is prepared for a variety of unknown future environments. But how does an individual cell determine its own fate? In a recent report, J. W. Veening et al. showed that the decision (to sporulate or not to sporulate) is not taken by the cell itself ― it is determined a few generations earlier, and the command is inherited as a “memory”.By making use of time-lapse microscopy, the authors followed the growth of individual cells and traced their history and lineage in microcolonies. Sporulation took place in two successive rounds, allowing a better use of resources. More surprising was the observation that the fate of many cells was already determined at the end of the exponential growth, long before any sporulation-related events could be detected.
 To assess which determinants were responsible for the sporulation decision, Veening et al. initially analysed cell aging (a process previously reported for other bacteria such as Caulobacter crescentus and Escherichia coli). Although they demonstrated that B. subtilis indeed suffered aging during growth, no correlation was found between cell age and spore formation. The authors studied other cell cycle-related physiological parameters but could not identify any relationships to the sporulation decision.
To assess which determinants were responsible for the sporulation decision, Veening et al. initially analysed cell aging (a process previously reported for other bacteria such as Caulobacter crescentus and Escherichia coli). Although they demonstrated that B. subtilis indeed suffered aging during growth, no correlation was found between cell age and spore formation. The authors studied other cell cycle-related physiological parameters but could not identify any relationships to the sporulation decision.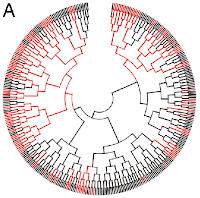 Nevertheless, spore formation did show certain lineage dependence ― i.e., families of cells were more likely to “agree” in their determination to become a spore or to stay as a vegetative cell. To visualize early events in the sporulation process, the authors examined recombinant B. subtilis strains that expressed green fluorescent protein (GFP) in response to active (phosphorylated) Spo0A. The latter protein is a key transcriptional regulator, directly responsible for the initiation of sporulation. In the reporter strains, GFP expression was highly lineage-dependent and could often be traced back for more than four generations. Therefore, the signal to activate Spo0A was apparently received from an ancestor, not as a message encoded in a DNA sequence but as an epigenetic inheritance. It is suggested that the sporulation signal is probably “memorized” by the autostimulatory architecture of the Spo0A regulatory cascade, which includes a number of kinases whose transcription is activated by phosphorylated Spo0A.
Nevertheless, spore formation did show certain lineage dependence ― i.e., families of cells were more likely to “agree” in their determination to become a spore or to stay as a vegetative cell. To visualize early events in the sporulation process, the authors examined recombinant B. subtilis strains that expressed green fluorescent protein (GFP) in response to active (phosphorylated) Spo0A. The latter protein is a key transcriptional regulator, directly responsible for the initiation of sporulation. In the reporter strains, GFP expression was highly lineage-dependent and could often be traced back for more than four generations. Therefore, the signal to activate Spo0A was apparently received from an ancestor, not as a message encoded in a DNA sequence but as an epigenetic inheritance. It is suggested that the sporulation signal is probably “memorized” by the autostimulatory architecture of the Spo0A regulatory cascade, which includes a number of kinases whose transcription is activated by phosphorylated Spo0A.
Epigenetic memory in bacteria can be a property of a genetic regulatory network (as in the reported example), but it can also be mediated by DNA methylation patterns. These mechanisms may play an important role in pathogenesis and the formation of socially organized structures such as biofilms and fruiting bodies.
Original article (open access):
Veening, J., Stewart, E.J., Berngruber, T.W., Taddei, F., Kuipers, O.P., Hamoen, L.W. (2008). Bet-hedging and epigenetic inheritance in bacterial cell development. Proceedings of the National Academy of Sciences USA, 105(11), 4393-4398. DOI: 10.1073/pnas.0700463105
Image sources:
First image from Wikipedia.
Second and third images from the PNAS original article (Copyright © 2008 by the National Academy of Sciences).

